3-N-Boc-aminocyclohexanone, also referred to as N-Boc-3-cyclohexanonemine or 3-(BOC-amino)cyclohexanone, is a vital organic intermediate that plays a critical role in modern chemical synthesis. With its Cas No.: 885280-38-6, molecular formula C₁₁H₁₉NO₃, and molecular weight of 213.27 g/mol, this compound stands out for its reliability and versatility in pharmaceutical and organic chemistry. It is typically supplied as a light beige solid with a purity standard of 98% or higher, ensuring consistency in industrial and laboratory applications. In this blog post, SACH, a high quality organic intermediate manufacturing factory, will share the role of Cas No.: 885280-38-6 3-N-Boc-aminocyclohexanone in pharmaceutical synthesis.
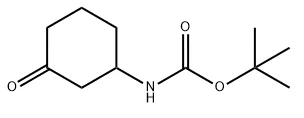
Key Physicochemical Properties of 3-N-Boc-aminocyclohexanone
Understanding the properties of 3-N-Boc-aminocyclohexanone helps scientists handle, process, and utilize the compound effectively.
* Appearance: Light beige solid
* Molecular Formula: C₁₁H₁₉NO₃
* Molecular Weight: 213.27 g/mol
* Melting Point: 81–85 °C
* Boiling Point: 335.9 ± 31.0 °C (predicted)
* Density: 1.06 ± 0.1 g/cm³ (predicted)
* Solubility: Slightly soluble in chloroform and methanol
* Acidity Coefficient (pKa): 12.01 ± 0.20 (predicted)
* Storage Conditions: Store in sealed, dry containers, away from light, ideally at -20 °C in a freezer
These parameters make N-Boc-3-cyclohexanone amine highly suitable for controlled synthesis pathways and ensure its long-term stability when stored under recommended conditions.
Synthetic Routes of 3-N-Boc-aminocyclohexanone
The synthesis of 3-(BOC-amino)cyclohexanone has been well established through multiple approaches. Two widely used methods are described below:
Method 1: Using 2-Cyclohexen-1-one and Tert-Butyl Carbamate
1. Dissolve 2-cyclohexen-1-one (150 mmol) and tert-butyl carbamate (145.11 mmol) in dichloromethane (DCM).
2. Add bismuth nitrate pentahydrate (28.8 mmol) and stir for 21 hours at room temperature.
3. Filter the mixture, wash the organic phase with sodium bicarbonate solution and saline, and dry.
4. Evaporate the solvent and purify by silica gel chromatography (EtOAc/cyclohexanol 3:7).
5. Obtain N-Boc-3-cyclohexanone amine in about 47% yield.
Method 2: Nucleophilic Substitution with 3-Cyclohexanone
* Starting materials: 3-cyclohexanone and tert-butyl carbamate.
* Boc groups are introduced via nucleophilic substitution reactions.
* Reported yield: ~55%.
* Detailed reaction steps can be found in *Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, issue 22, pp. 6118–6122*.
Both methods confirm that 3-N-Boc-aminocyclohexanone can be reliably synthesized, with slight variations in yield depending on reaction conditions.
Applications of 3-N-Boc-aminocyclohexanone in Drug Synthesis
One of the most important uses of 3-N-Boc-aminocyclohexanone is as a pharmaceutical intermediate.
BTK Inhibitors and Cancer Therapy
The compound serves as a key building block in synthesizing BTK (Bruton’s Tyrosine Kinase) inhibitors, which are frontline drug candidates for treating:
* Autoimmune diseases
* Chronic lymphocytic leukemia (CLL)
* Mantle cell lymphoma (MCL)
* Other cancers where BTK signaling plays a pathological role
By acting as a precursor, N-Boc-3-cyclohexanone amine ensures high efficiency and stability during the multi-step synthesis of these advanced therapies.
Organic Synthesis and Boc Protection
In addition to pharmaceuticals, 3-(BOC-amino)cyclohexanone is widely used in organic synthesis. The BOC protective group stabilizes amino functionalities by shielding them from oxidation, decomposition, or side reactions. This makes the compound an ideal intermediate in complex molecule synthesis, including peptides and heterocyclic compounds.
Safety and Handling Guidelines for 3-N-Boc-aminocyclohexanone
Like many organic intermediates, 3-N-Boc-aminocyclohexanone requires careful handling.
* Hazard Statements:
* H302: Harmful if swallowed
* H315: Causes skin irritation
* H319: Causes serious eye irritation
* H335: May cause respiratory irritation
* Precautions:
* Avoid direct skin and eye contact
* Use protective gloves, goggles, and masks
* Ensure good ventilation during handling
* Wash thoroughly after use
* Storage Recommendations:
* Keep sealed in a dry, dark environment
* Store under -20 °C to ensure stability
* Avoid prolonged exposure to heat or moisture
Adhering to these safety protocols ensures that laboratories and industrial facilities can work with N-Boc-3-cyclohexanonemine without compromising worker health or product integrity.
Packaging and Supply Information for 3-N-Boc-aminocyclohexanone
Commercial suppliers typically offer 3-N-Boc-aminocyclohexanone under the following specifications:
* Minimum Order: 1 kg
* Standard Purity: ≥98%
* Packaging: 25 kg per drum
* Form: Light beige solid
Such packaging is designed to maintain product quality during transportation and long-term storage. Bulk availability ensures that pharmaceutical companies and research institutions can secure a stable supply for ongoing projects.
Why Choose 3-N-Boc-aminocyclohexanone as a Research Intermediate?
Several features make 3-(BOC-amino)cyclohexanone a preferred intermediate:
1. High Purity: With standard quality ≥98%, it ensures reproducible results in synthesis.
2. Versatile Applications: Essential in drug discovery and organic chemistry.
3. BOC Stability: Protects amino groups, reducing unwanted side reactions.
4. Proven Synthetic Routes: Reliable preparation methods with good yields.
5. Industry Demand: Growing importance in the pharmaceutical sector, particularly in oncology research.
These attributes make Cas No.: 885280-38-6 not just another intermediate but a strategic compound in advancing drug development pipelines.
Conclusion
3-N-Boc-aminocyclohexanone (Cas No.: 885280-38-6) is more than a chemical intermediate—it is a cornerstone in pharmaceutical innovation. With its precise physicochemical properties, reliable synthetic methods, and vital role in BTK inhibitor development, it contributes significantly to advancing therapies for cancer and autoimmune disorders.
Its stability, versatility, and consistent quality make N-Boc-3-cyclohexanonemine indispensable in both research laboratories and industrial pharmaceutical manufacturing. As global demand for advanced drug intermediates continues to rise, 3-(BOC-amino)cyclohexanone is positioned as a critical enabler of future medical breakthroughs.
www.hzsqchem.com
SACH